This study presents an in-depth analysis of the potential application of vitrification within an isochoric chamber, with specific attention paid to various parameters such as materials used, temperature, solution type and concentration, and thermodynamic constraints like melting point and critical concentration. The aim is to determine the influence of these factors on the occurrence of partial ice-vitrification. The study critically addresses the potential formation of cavities within the chamber due to the differing contraction of the solution and the chamber itself, a factor that complicates the detection of vitrification based solely on pressure data.
The study proposes that positive pressure within the chamber forms only when the cavity is eliminated or filled. This can happen through the expansion of ice, which in turn ripens the remaining solution and possibly leads to partial vitrification. The article’s goal is to identify thermodynamic limits on the amount of undetected ice that could form in the chamber, and to quantify the conservative effects of this ice formation on driving partial vitrification, considering temperature and starting concentration.
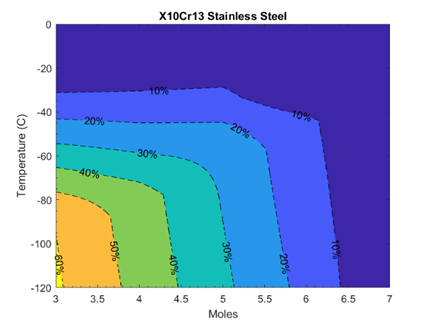
A simplified spherical isochoric chamber serves as the control volume, and a binary solution of water and pure DMSO is used at varying concentrations. Additionally, the article examines the impact of different chamber materials (including X10Cr13 Stainless Steel, Aluminum 7075, Tungsten, and ABS) with varying thermal contraction coefficients on the cavity volume and partial vitrification processes. This research contributes to a more comprehensive understanding of the complex dynamics involved in the vitrification process in an isochoric chamber.