This study introduces a novel approach to the challenge of supercooled biopreservation, acknowledging the inherent risk of random ice nucleation due to the stochastic nature of ice formation from a supercooled solution. We present a statistical model of stochastic ice nucleation, demonstrating the interconnection between potential reduction in metabolic rate and supercooling stability, the latter being the likelihood of ice nucleation. A quantitative approach is developed to balance supercooling stability against potential metabolic reduction, examining how the stability-metabolism relationship varies with system size under different modes of nucleation.
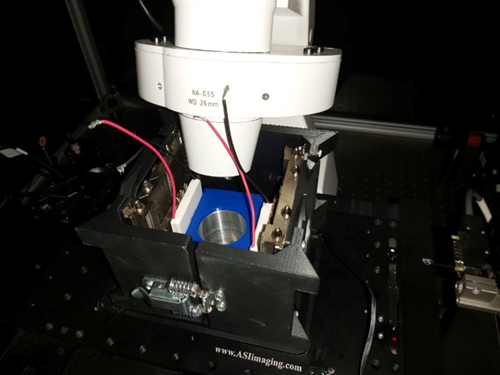
In addition, we propose the design of a microscope platform that employs the principles of biopreservation and biothermodynamics. This design allows for the examination of cellular metabolism at supercooled temperatures via fluorescence, extending previous techniques probing the temperature dependence of cellular metabolism from 37°C down to subzero temperatures. We describe a device using two peltier elements to control the cooling and heating of a 35mm glass petri dish on a microscope stage, enabling an unprecedented exploration of metabolic dependence at temperatures below 0°C, a concept fundamental to biopreservation. This work represents an important step forward in understanding and optimizing the process of biopreservation.